A patient’s tumor profile has the potential to guide precision oncology care, but only if the results are reported in time.
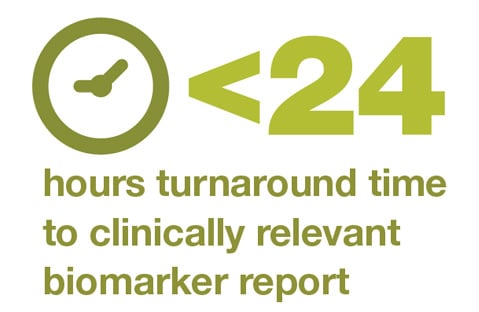
The Ion Torrent™ Oncomine™ Dx Express Test on the Ion Torrent™ Genexus™ Dx System enables fast NGS testing for clinically relevant biomarkers in as little as 24 hours. This specimen-to-report workflow is easy to implement and operate with minimal hands-on time. Watch our video below to learn more.
NEW! Webinar On Demand
The development of targeted therapies has led to a paradigm shift in the treatment of NSCLC. However, timely genomic profiling is the cornerstone of realizing the potential of precision oncology.
In this recorded Industry Satellite Symposium from the European Lung Cancer Congress (ELCC) 2024, you will:
The Oncomine Dx Express Test can deliver results in less than 24 hours, allowing the laboratory to combine the molecular biomarker results with the immunohistochemistry and provide one combined report to aid clinicians in making therapy decisions.
Learn more about the importance of timely availability of genomic profiling for oncology patients.
Oncomine Dx Express Test detects clinically relevant biomarkers per professional medical guidelines1,2, including ESCAT for clinically relevant biomarkers and approved therapies.
Oncomine Dx Express Test detects substitutions, insertions and deletions, copy number variants, and rearrangements or fusions across 46 genes including EGFR, BRAF, KRAS, ERBB2, MET, ALK, ROS1, RET, MET, and NTRK1/2/3, among others.
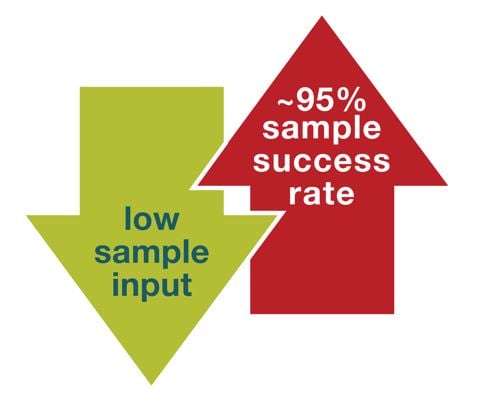
Oncomine Dx Express Test requires only 10 ng of DNA and RNA extracted from as little as two 5-micron FFPE slides, maximizing results from limited tissue and small biopsies. Plasma from liquid biopsy provides an additional sample type.
The Genexus System workflow is easy to integrate NGS into any pathology laboratory. With automated library preparation, sequencing, analysis, and reporting involving as little as 20 minutes of hands-on time and a single IVD software it reduces laboratory staff burden, the potential for human errors, and alleviates the need for bioinformatics expertise.
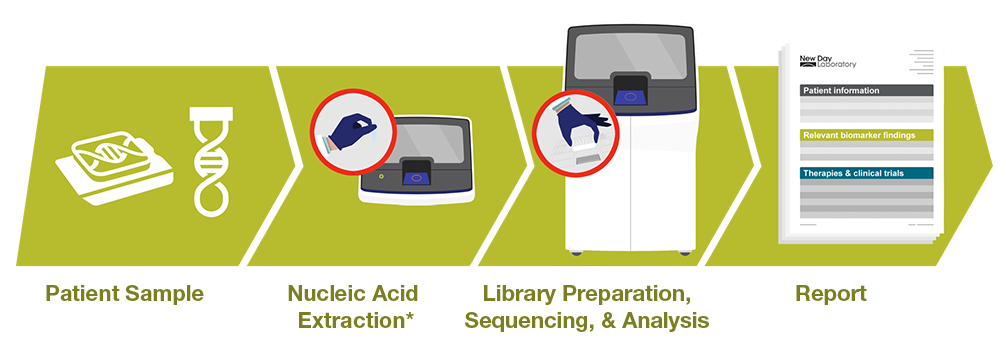

Oncomine Reporter Dx software helps users deliver a patient sample-specific summary of each biomarker matched with relevant therapies, guidelines, clinical trials and peer-reviewed literature.
¹100% clinically relevant biomarkers = unique biomarkers that have an ESCAT level of IA, IB, or IC; majority = 86%, BRCA1/2 and MSI-H are not covered by the Oncomine Dx Express Test.
² Mosele F. et al. (2020) Ann Oncol 31:1491
For In Vitro Diagnostic Use. CE-IVD according to IVDD. Not available in all countries including the United States. The content provided herein may relate to products or workflows that have not been officially released or fully validated and is subject to change without notice. © 2022 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.
Abbreviated Intended Use: The Oncomine Dx Express Test is a qualitative in vitro diagnostic test that uses targeted next-generation sequencing (NGS) technology, the Ion Torrent Genexus Dx System to detect deletions, insertions, substitutions and copy number gain present in 42 genes and fusions in 18 genes from DNA and RNA extracted from formalin-fixed, paraffin-embedded (FFPE) tumor tissue samples. Oncomine Dx Express Test also detects deletions, insertions, substitutions in 42 genes and fusions in 7 genes from cfTNA extracted from plasma samples. The Oncomine Dx Express Test is intended to provide clinically relevant tumor mutation profiling information to be used by qualified health care professionals in accordance with professional guidelines as an aid in therapy management of cancer patients with solid malignant neoplasms using FFPE samples and as an aid in therapy management of cancer patients with non-small cell lung cancer using plasma samples. It is not conclusive or prescriptive for labeled use of any specific therapeutic product.
We've detected your location to be Japan.
Sorry, you cannot access this website. The content on www.oncomine.com is only intended for healthcare professionals. Formore information on our research solutions, please visit ThermoFisher.com
このウェブサイトは、日本国内の医療関係者の方への情報提供を目的としており、一般の方に対する情報提供を目的としたものではないことをご了承ください。研究用製品の情報はThermoFisher.comよりご覧ください。